Welcoming the 2024 NOMIS–ETH Fellows
This October, the Centre for Origin and Prevalence of Life at ETH Zürich welcomed three new postdoctoral fellows as part of the NOMIS–ETH Fellowship Programme. Sean Jordan, Taylor Priest and David Schnettler each explore critical aspects of the origin of life, its evolution and place in the broader cosmic context.

Whether searching for life on distant exoplanets, studying genetic exchange across Earth’s biomes or probing the primordial mechanisms of molecular replication, the three early-stage researchers are breaking new ground in the field of the origin and prevalence of life. The NOMIS–ETH Fellowship Programme will support them to pursue their own research projects for a duration of 3+1 years, while benefitting from the infrastructure and interdisciplinary networking opportunities offered by the COPL.
The welcome event was opened by Jörg Heldmann, Deputy Managing Director of the NOMIS Foundation, and Didier Queloz, Director of the Centre for Origin and Prevalence of Life at ETH Zurich, and gave the new fellows the opportunity to present their research. Coming from backgrounds in astrophysics, microbiology, and biochemistry respectively, their projects have very different focuses and, at the same time, share a common interest in investigating essential mechanisms that underpin life.
Sean Jordan: Investigating the habitability of planets beyond Earth
Dr. Sean Jordan began his presentation by painting a vivid picture of our neighbouring planet, Venus. Similar in size and composition, it once seemed plausible that Venus, like Earth, might have supported life in its distant past. However, when space exploration reached Venus in the latter half of the 20th century, it revealed a far different reality – a planet shrouded in clouds of concentrated sulfuric acid and surface temperatures high enough to melt lead. Venus, it seemed, was a picture of hell on Earth.
How did Venus become so hostile, and did it ever resemble the Earth in its distant past? As Jordan explained, this question remains unanswered and has profound implications for the habitability of Earth-like planets beyond the Solar System.
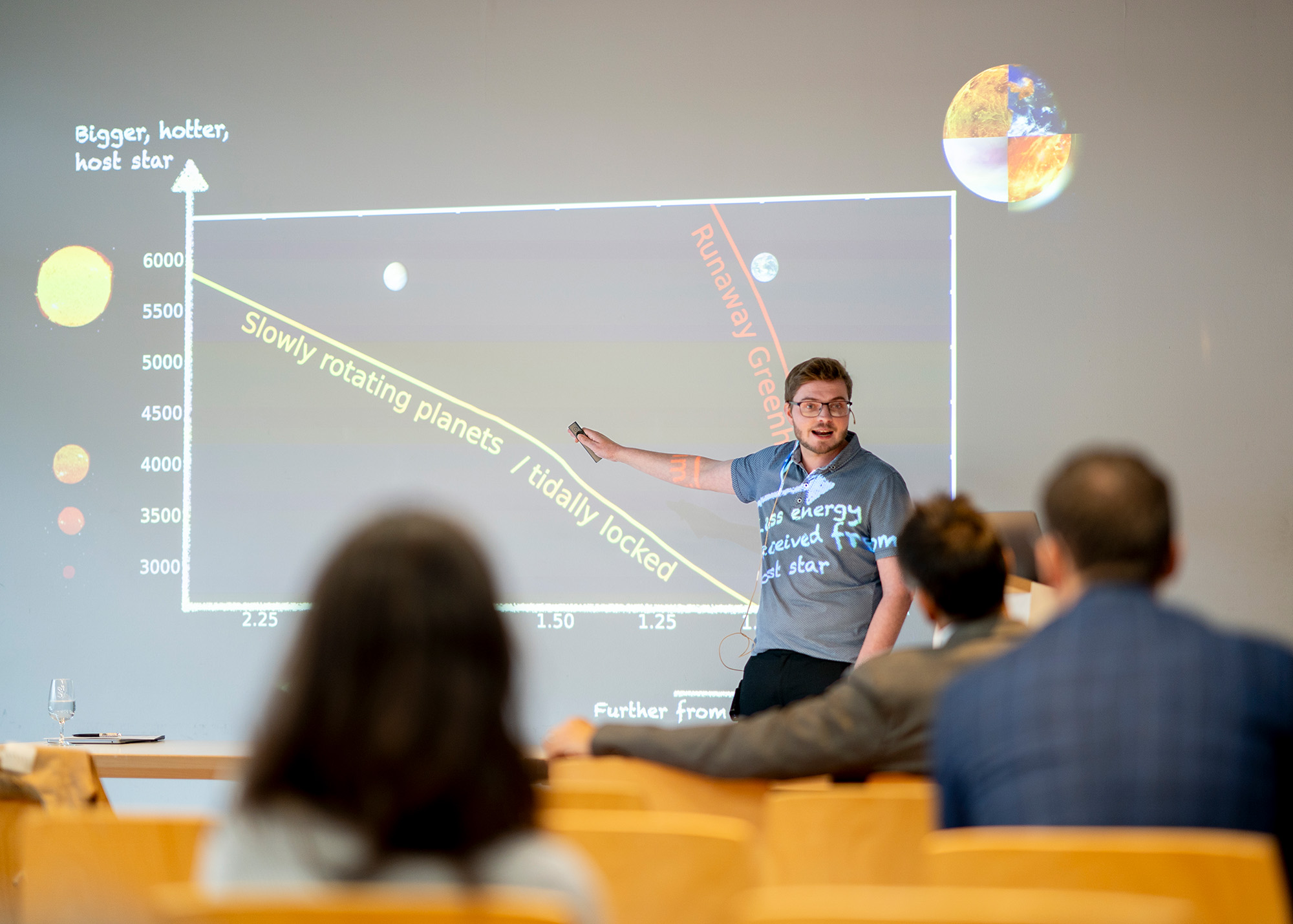
Today exoplanet astronomy provides the opportunity to test what mechanisms gave rise to this dramatic dichotomy between Venus and the Earth, and what controls the habitability of rocky exoplanets more generally. In his research, Sean Jordan is investigating how planetary paradigms represented locally by Venus and the Earth can be used to identify habitable versus uninhabitable rocky exoplanets in orbit around distant stars.
His research does not stop with Venus. By comparing what we know about Venus and Earth, Jordan aims to develop a framework that can be applied to exoplanets: “In this way, we can begin to constrain the number of habitable rocky exoplanets that may exist in the universe, and test whether the Earth itself is unique, whether the origin of life on Earth was unique, or whether life emerges inevitably on every habitable planet that we come across”, he explained.
Dr. Sean Jordan will carry out this research in the Department of Physics (D-PHYS) in the Exoplanets and Habitability, under the mentorship of Prof. Sascha Quanz.
Further reading:
Jordan, S. et al. "Photochemistry of Venus-like Planets Orbiting K- and M-dwarf Stars." The Astrophysical Journal, 922, 44. external page https://iopscience.iop.org/article/10.3847/1538-4357/ac1d46
Taylor Priest: Tracking the role of mobile genetic elements in evolution
Dr. Taylor Priest introduced the audience to a generally lesser-known yet crucial force in the evolution of life: mobile genetic elements (MGEs). In contrast to the vertical transfer of genetic information in living organisms from a parent to a child generation, these small, mobile segments of genetic material move horizontally between different organisms, shuffling genes and contributing to genetic diversity – one of the central mechanisms through which life has evolved over the past four billion years.

Priest described how MGEs can be found in almost every environment on Earth, where they facilitate the adaption of organisms to new conditions and drive their evolution. His research combines advanced computational analysis of DNA sequence information obtained from diverse environments across the planet with experimental techniques and fieldwork, to map the distribution and influence of these elements on the ecology and evolution of life under different conditions on Earth.
In his research, Taylor Priest is focused on understanding the diversity of MGEs that exist and the genetic material that they carry within and across environments. By further connecting MGEs to the organisms that they associated with, through computational and experimental work, he hopes to provide new insights into the mechanisms that drive diversification and evolution of microbial life on Earth. His work has the potential to inform both our understanding of natural evolutionary processes and synthetic biology applications, where MGEs could be used to engineer new biological systems.
Dr. Taylor Priest will conduct his research in the Department of Biology (D-BIOL) in the Microbiome Research Lab, under the mentorship of Prof. Shinichi Sunagawa, and in the Department of Environmental Systems Science, under the mentorship of Prof. Marie C. Schoelmerich.
Further reading:
Koonin, E. V., (2016). “Viruses and mobile elements as drivers of evolutionary transitions” Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1701). external page doi.org/10.1098/rstb.2015.0442
Arnold, B. J., et al., (2022). “Horizontal gene transfer and adaptive evolution in bacteria” Nature Reviews Microbiology 20(4). external page doi.org/10.1038/s41579-021-00650-4
David Schnettler: Exploring the origins of life’s molecular machinery
Dr. David Schnettler is tackling a fundamental question about life’s early development: how did the first molecules responsible for replicating genetic material evolve? Today, sophisticated enzymes called polymerases perform this task with incredible precision, but their complexity raises questions about how the first versions of these molecules might have looked.

Schnettler’s work builds on the Dayhoff hypothesis, which suggests that modern, complex proteins may have evolved from much simpler peptides. Using cutting-edge protein engineering techniques, he is working to experimentally recreate these early forms of life’s molecular machinery. As he explained during his presentation, “At the origin of life, the replication of early genetic polymers poses a hen-egg conundrum. So, how did the first polymerases look like?”
By piecing together how primitive proteins might have functioned, David Schnettler’s research intends to experimentally address this question using recently developed tools from the field of protein engineering, and thus opening new doors to understanding the earliest stages of life on Earth.
Dr. David Schnettler will conduct his research in the Department of Biosystems Science and Engineering (D-BSSE) in the Bioprocess Laboratory, under the mentorship of Prof. Sven Panke.
Further reading:
Schnettler, J. D. et al. "Selection of a promiscuous minimalist cAMP phosphodiesterase from a library of de novo designed proteins." Nat. Chem. 1–9 (2024) external page doi:10.1038/s41557-024-01490-4.
(Showing that a relatively short mini-protein without evolutionary history, identified by high-throughput screening, is capable of catalyzing a fundamental biochemical reaction with high proficiency)
 Didier Queloz, Director of the Centre for Origin and Prevalence of Life at ETH Zurich, opening the welcome event of the NOMIS–ETH Fellows, Cohort 2024. (Image: Peter Pedersen / COPL)
Didier Queloz, Director of the Centre for Origin and Prevalence of Life at ETH Zurich, opening the welcome event of the NOMIS–ETH Fellows, Cohort 2024. (Image: Peter Pedersen / COPL) Jörg Heldmann, Deputy Managing Director of the NOMIS Foundation, introducing the NOMIS–ETH postdoctoral fellows. (Image: Peter Pedersen / COPL)
Jörg Heldmann, Deputy Managing Director of the NOMIS Foundation, introducing the NOMIS–ETH postdoctoral fellows. (Image: Peter Pedersen / COPL) Audience at the welcome event of the NOMIS–ETH Fellows, Cohort 2024. (Image: Peter Pedersen / COPL)
Audience at the welcome event of the NOMIS–ETH Fellows, Cohort 2024. (Image: Peter Pedersen / COPL) Q&A session during the welcome event of the NOMIS–ETH Fellows, Cohort 2024. (Image: Peter Pedersen / COPL)
Q&A session during the welcome event of the NOMIS–ETH Fellows, Cohort 2024. (Image: Peter Pedersen / COPL) Q&A session during the welcome event of the NOMIS–ETH Fellows, Cohort 2024. (Image: Peter Pedersen / COPL)
Q&A session during the welcome event of the NOMIS–ETH Fellows, Cohort 2024. (Image: Peter Pedersen / COPL) The NOMIS–ETH Fellows. (Image: Peter Pedersen / COPL)
The NOMIS–ETH Fellows. (Image: Peter Pedersen / COPL)