Research Project P. Sossi, N. Küter, M. Thompson
Experimental constraints on photochemical pathways to life-essential precursors in the atmospheres of rocky planets

The ingredients for the origin of life may have had humble beginnings as gaseous molecules, such as methane, in the atmosphere of the early Earth and on other planets. Their subsequent destruction by stellar ultraviolet light can produce a bevy of new molecules and, in so doing, potentially provide the feedstocks for prebiotic reactions to proceed [Fig. 1]. Here, we aim to experimentally quantify the identities of, and the rates at which these photochemical reactions occur, and whether isotopic traces of the existence of such molecules can be detected in ancient rocks on Earth.
Abstract
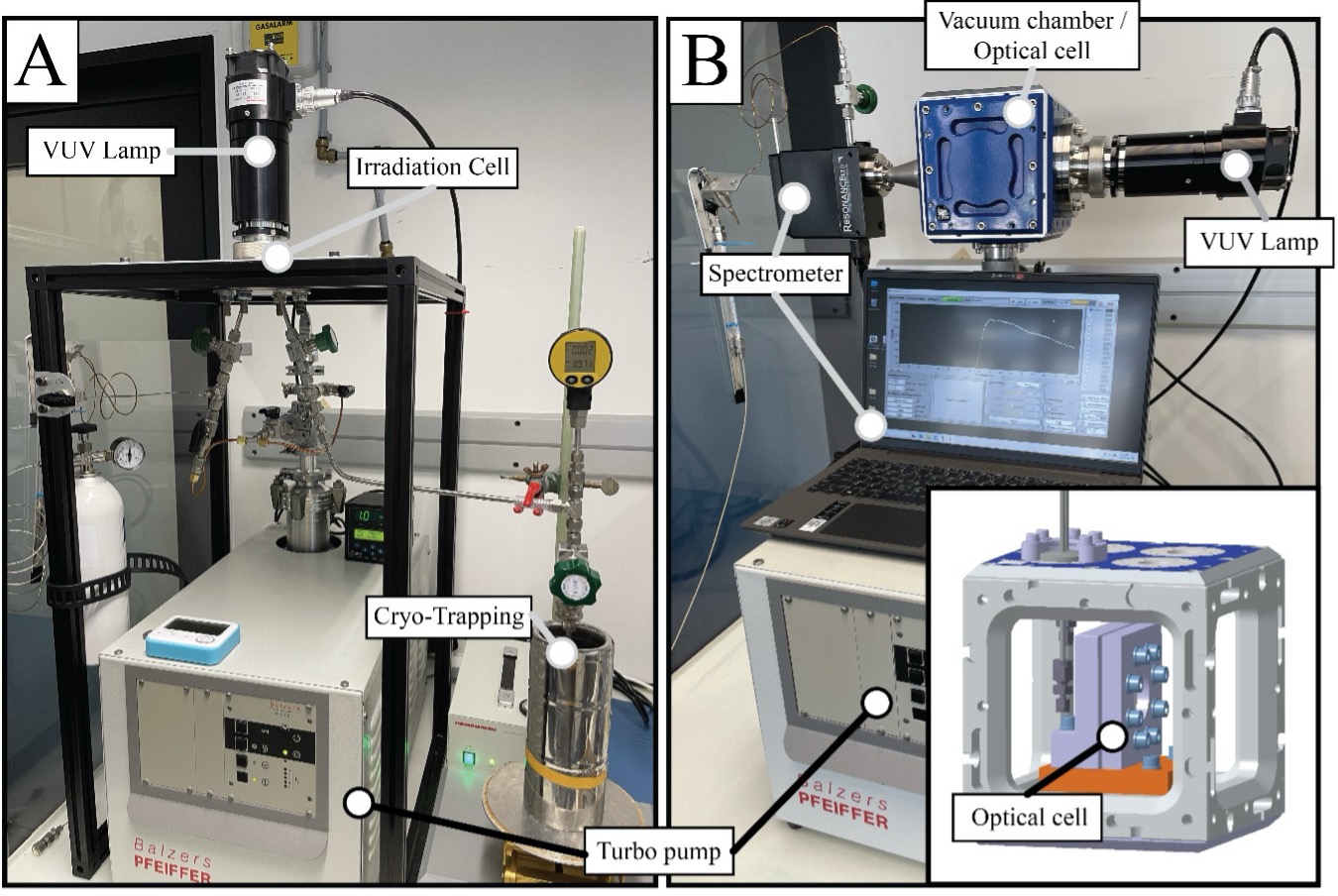
The atmosphere of the early Earth, as well as those of other rocky planets, was initially composed primarily of simple molecules, such as H2, N2, CO2, H2O and CH4. However, depending on the amount of stellar ultraviolet radiation the planet receives, these molecules absorb photons that lead to i) the creation of new molecules and radicals that can, in turn, react with one another and ii) the fractionation of the isotopic abundances of their constituent elements between the parent- and daughter products. However, the rates at which these molecules undergo photolytic destruction, and the isotopic fractionation that attends it, are poorly known, especially over a wide range of temperatures. Here, we aim to constrain photochemical reactions occurring in the vacuum-ultraviolet (VUV, 10 – 200 nm) range during the irradiation of a key molecule, CH4, which is thought to have been present at parts-per million levels on the early Earth and that comprises 5.65 % of Titan’s atmosphere. To do so, we will develop a photochemical experiment in which a controlled atmosphere of CH4 undergoes VUV irradiation, and the abundances and identities of the resulting products quantified through a combination of spectroscopy and mass spectrometry [Fig. 2]. This approach will permit the characterisation of the VUV cross section of CH4 and its intermediate products, as well as the isotopic fractionation factors between them. These fundamental data will be implemented into numerical models of photochemical reactions to understand the stability of putative life-essential precursor molecules in planetary atmospheres, as well as to detect any isotopic tracers in Archean rocks of the role of photochemical reactions as energy sources for the earliest organisms.
Team
PI: Prof. Paolo Sossi (ETH, Department of Earth and Planetary Sciences)
Co-Is: Dr. Nico Küter (ETH, Department of Earth and Planetary Sciences), Dr. Maggie Thompson (ETH, Department of Earth and Planetary Sciences)
Collaborators: Cara Magnabosco (ETH, Department of Earth and Planetary Sciences), Thomas Drant (Paris-Saclay/LMU), Josh Krissansen-Totton (U. Washington), Nick Wogan (NASA Ames), Patrick Hemberger (PSI), András Bodi (PSI), Naizhong Zhang (EMPA), Joachim Mohn (EMPA), Stefano Bernasconi (ETH, Department of Earth and Planetary Sciences)